Schoffelen & Plasqui explained the two-century old history of whole-body energy metabolism measurement in their 2017 paper.
Introduction
Indirect calorimetry calculates the total energy expenditure (EE) of the human body by estimating the amounts of substrates oxidized by measuring gas exchange, such as O2 consumption and CO2 production. The total amount of EE is then calculated by summing the energy density of substrates with a known quantity. “Indirect calorimetry” is fundamentally different from “direct calorimetry,” which tracks the body’s direct heat loss. A multitude of elements, including nutritional composition, oxidation, energy released as heat and work, and gas exchange, had to be learnt and combined before indirect calorimetry could be used to measure EE in humans.
Even though the bulk of current systems are based on direct calorimetry, one could argue that it is the gold standard for measuring energy efficiency (EE) because no “indirect” conversion factors are necessary to get EE values from chemical energy stored in substrates. Energy can be changed, transmitted, and stored in at least as many diverse ways as it can take on a variety of shapes. Examples include energy that appears as motion, heat, and radiation (light). For instance, mechanical or chemical processes can move and change energy from one form into another. As a result, the second important difference between direct and indirect calorimetry is that, while direct calorimetry measures heat dissipated by the body, indirect calorimetry measures total EE, which is frequently not entirely released as heat from the body. A part of the body’s total energy expenditure during exercise is used to complete this “external task” and is not released as heat. This disparity is well-expressed in the Webb paper “The effort of walking: a calorimetric study” (Webb et al. 1988). Both directly and indirectly, using gas exchange and a suit calorimeter, the subjects’ EE was evaluated as they walked on a treadmill. It was shown that some of the body’s energy was used up while walking rather than being released as heat. Please be aware that energy measurements only take into account work that has been converted to heat inside of a direct calorimeter, and that energy from other sources (such lights, fans, air conditioning, televisions, and computers) must be subtracted. Additionally, indirect calorimetry finds changes in gas composition that are not caused by the body (carbonated drinks, burning cigarettes, and flushing toilet).
Historical background throughout the ages
In “On Youth and Old Age, On Life and Death, On Breathing,” Aristotle discussed breathing. He blended the terms “internal fire” and “breathing.” Around 360 B.C., Plato first mentioned breathing in his work “Timaeus” (Aristotle and Ogle 1897). In reaction to the perplexing connection between food, energy, and breathing, Aristotle said, “Moreover, in what sense are we to accept this ludicrous notion, that heat is created out of the breath?” Because we see heat coming from food and not from the air (Aristotle and Ogle 1897). Given the constraints of time, this is the most effective way to describe the fundamentals of both direct and indirect calorimetry.
In 1614, Santorio Sanctorius noticed a difference between the weights of all consumed foods and excretions. He referred to this at the time puzzling disparity as “insensible sweat.” Sanctorius was virtually the first to prove that CO2 was the weight of carbon leaving the body, although he was at that point unable to explain it.
Between the years of 1774 and 1777, Antoine-Laurent Lavoisier and his wife and colleague made important strides in the study of human and animal respiration in the wake of Priestly’s finding that oxygen in the air was not a constant. (1777; Lavoisier). They were able to detect oxygen consumption and carbon dioxide production despite being unaware of substrate oxidation and its conversion to EE (Fig. 1). (1888 Grimaux).

Fig. 1
Lavoisier was measuring a subject’s breathing when they were at rest, as his wife illustrated. Adopted from: The Wellcome Library in London (Grimaux 1888)
Shortly after, in 1780, Lavoisier and Laplace published instructions on how to use the well-known “ice-calorimeter,” which involved placing an animal inside of a sealed, insulated space that was cooled with ice in order to determine its heat production (direct calorimetry) from the amount of meltwater produced. Therefore, even though the indirect calorimeter’s gas-exchange findings could not yet be converted into EE, Lavoisier probably built the first direct and indirect calorimeters. “Respiration is merely a slow combustion of carbon and hydrogen, analogous in every respect to that which occurs in a lamp or an illuminated tree,” claimed Lavoisier. “From this standpoint, the animals that breathe are actually combustible bodies that burn and devour themselves.” or “Animals that breathe are true combustible bodies that burn and devour,” showing that the heat created by a living object is comparable to the chemical process of burning food in fire, albeit at a slower rate (Fig. 1), and that breathing is simply a slow combustion of carbon and hydrogen. This combustion is identical to that which occurs in a lamp or a lit candle in every way (Lavoisier 1783).
The scientific understanding of physics, chemistry, and food composition had improved about a century after Lavoisier by the late 19th century, to the point where Pettenkofer in 1862 described how to build the first whole-room open-circuit indirect calorimeter (Fig. 2). 1860 (Pettenkofer).
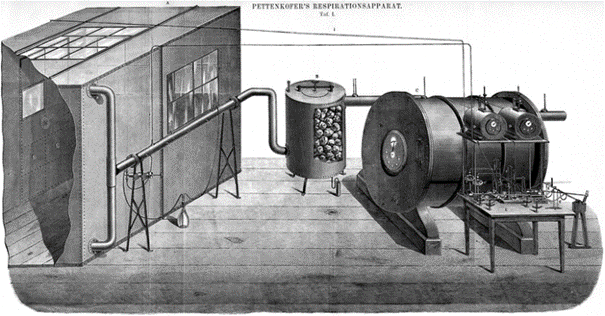
Fig. 2
Pettenkofer’s Repirationsapparat was the first whole-room open-circuit indirect calorimeter for humans. Adopted from: “Lehrbuch Besanez’s der Physiologischen Chemie” by Eugen von Gorup (Gorup-Besanez 1867)
Substrate oxidation information from Magnus-Levy (1893), Zuntz and Schumburg (1901b), and Zuntz and Lehmann (1890) became available at the turn of the century. ” The “Haldane transformation” or “Haldane correction” is a crucial concept about the conservation of inert gases within or going through a calorimeter. The body does not use the inert gases found in fresh air, which are primarily nitrogen (78%) and trace amounts of noble gases like argon and helium (0.9%). Unless RER = 1, oxygen consumption and carbon dioxide generation are typically not equal, meaning that breathing volume in and out are not equal. Volume in and volume out for inert gasses remain equal, according to Haldane, and this can be used to determine changes in breathing volume out from in or vice versa.
As a result, the Haldane adjustment, which is still employed in indirect calorimeters today, enables the attainment of almost perfect balance with regard to in-flow and out-flow. A method for chemical analysis of gas composition was also exhaustively detailed by Haldane in 1898 (Haldane 1898), and Scholander’s approach from 1947 (Scholander 1947) and revisions were still in use in the 1980s (Fig. 3). (McLean and Watts 1976; Webb et al. 1988; Schoffelen 1985).
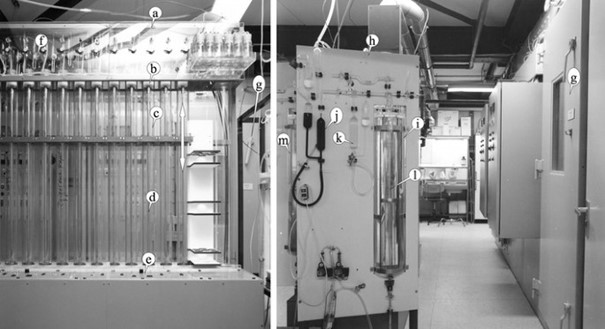
Fig. 3
The Maastricht University used a respiration chamber and chemical gas analysis equipment in the 1980s. An automated sample unit (a) on the left panel uses controlled spindles to move Teflon and mercury-sealed pistons (b) up and down (arrow) in precision glass tubes (c) (d). Momentary sampling, metal-glass sealing, and faultless storage of gas samples obtained from the respiration chambers were all made possible by the control of timing (e) and solenoid valves (f) (g). A modified Haldane chemical gas analysis device is shown in the right panel (h). When using a three-sample analysis, its precision dual cuvettes in a water container I allowed analysis to 0.001% absolute for CO2 and O2. It functions by flushing the sample cuvette with O2 absorbent (j) and CO2 absorbent (k) to remove O2 and CO2, then measuring the change in gas volume that results (l). For continuous measurements lasting more than 24 hours, two units were required, and the second unit (m) is visible behind the front unit.
In 1904, Atwater added closed-circuit gas-exchange measurements to a whole-room direct calorimeter (Atwater and Benedict 1905). According to Mclean and Tobin, this combination of direct- and closed-circuit indirect calorimetry “was a landmark in human calorimetry” (McLean and Tobin 1987). There are many more era pioneers left now.
Researchers were able to begin more extensive studies on the relationship between work, heat, and respiration in both animals and people well into the 19th century (Pettenkofer 1862; Ott and Foster 1891; Haldane 1892; Atwater et al. 1897; Marcet et al. 1898; Zuntz and Schumburg 1901b; Atwater and Benedict 1905; Hagemann 1911; Langworthy and Milner 1911; Hill and Hill 1914). Importantly, the components of food were examined, and their chemical reactions with oxygen to produce carbon dioxide and energy were determined (Zuntz and Schumburg 1901b; Magnus-Levy 1893) from three fundamental substrates: fat, protein, and carbs (Table 1).
Table 1 O2 consumption (l), CO2 production (l), and EE (kcal) per gram of substrate used (i.e., carbohydrate, protein, and fat), including correction of EE (kcal) per gram of urinary nitrogen katabolized
| Substrate 1 g | O2 uptake (l) | CO2 production (l) | EE (kcal) | EE (kcal) |
| Carbohydrate | 0.8288 | 0.8288 | 4.182 | Zuntz 1897 (Zuntz and Schumburg 1901b) |
| Protein | 0.967 | 0.7752 | 4.316 | Loewy-Lusk 1928 (Lusk 1928) |
| N2-urine katabolized | – | – | − 2.17 | Weir (1949) |
| Fat | 20.193 | 14.311 | 9.461 | Cathcart 1931 (Cathcart and Cuthbertson 1931) |
This made it possible to calculate the human EE from gas exchange, taking into account the variable carbon dioxide generation contribution and the adjustment for energy loss of N2 molecules in urine (Table 1). (Schoffelen and Plasqui 2016; Livesey and Elia 1988; Consolazio et al. 1963; Brouwer 1957; Weir 1949; Zuntz and Schumburg 1901b). One of the resulting formulas was expressed as EE [kcal] = 3.941 ⋅ O2 [l] + 1.106 ⋅ CO2 [l] −2.17 ⋅ N2 [g].EE [kcal] = 3.941 ⋅ O2 [l] + 1.106 ⋅ CO2 [l] −2.17 ⋅ N2 [g].
It is not necessary to measure heat for this computation of EE from gas exchange. It is entirely based on measured gas amounts for the amount of carbon dioxide produced and oxygen absorbed. This method based on gas exchange became known as “indirect calorimetry” as there was no “direct” way to detect heat.
Measuring human EE with direct- and indirect calorimeters remained difficult for the most of the 20th century. Electronic devices like computers weren’t yet made or were just in the prototype stage. In contrast to sensors, which were manually documented or plotted using chart recorders, gasses needed to be analyzed by trained technicians using chemical techniques to determine values for a single gas sample at a time (Scholander 1947; Haldane 1898).
These well-known research organizations produced truly excellent work that is largely still applicable today. They validated methods for heat- (direct) and gas-exchange (indirect) measurements, measured the caloric values of the macronutrients protein, carbohydrates, and fat, and developed formulas for calculating energy expenditure (EE) from gas-exchange and measured urinary nitrogen (Harris et al. 1919; Lusk 1917; Zuntz and Schumburg 1901b; Haldane 1892; Pettenkofer 1862; Webb 1985).
The first mini- and micro-computers of the 1970s were a result of the equipment revolution that began in the 1960s with advances in electronics. The first research teams to take advantage of these advancements were those focused on Human EE in Cambridge and Lausanne. Within a decade, many other research teams did the The development of commercial metabolic carts, which brought ventilated hood diluted flow indirect calorimetry to the bedside, hastened this rise in indirect calorimetry. Despite the fact that this expansion and technical advancements did not alter the fundamental calorimetric principles in use, the field of human indirect calorimetry was greatly broadened.
The last operational whole-body direct calorimeter may be the re-engineered Snellen air-flow calorimeter in Ottawa, Canada. Recent literature did not list any operational larger whole-room calorimeters with both direct- and indirect calorimetry; they all appear to have been decommissioned. Whole-room calorimeters with indirect calorimetry, on the other hand, have both been newly built and partially operational for some time. Around 12–15 functioning whole-room indirect calorimeters are estimated from the literature, and another 10–15 are known to be in use, effectively steadying 20–30 sites globally.
The pursuit for fast dynamic response, offering a high degree of short interval and whole-range accuracy, is a trend in whole-room indirect calorimetry that has yet to reach its full potential and become a widespread use. Whole-room indirect calorimeters are known for having a long response time since their accuracy depends on multiplying it by the accuracy of gas analysis with a huge volume, which makes them difficult to use for extremely short interval evaluation. As a result of technological advancements and computers, evaluations typically last 15 to 30 minutes (Webb et al. 1988; Nguyen et al. 2003; Henning et al. 1996; Tokuyama et al. 2009), and accuracy for low-level EE is still reliant on the individual implementation’s minimum floor-level noise (Murgatroyd et al. 1987). Individual implementation may result in unfiltered noise levels in gas-exchange values ranging from 50 to 1000 ml min1 peak to peak (personal observation and communication), and the search for fast dynamic response has primarily been focused on mathematical procedures for re-evaluation of measured results, or noise reduction (Tokuyama et al. 2009; Granato et al. 2004).
It is hypothesized that the next development in indirect calorimetry would integrate increased measurement accuracy for volumetric and gas analysis elements with mathematical handling of the smallest finite-step assessment. The traditional emphasis on physics must be merged with contemporary computer technology to complete the next stage; that is, the current emphasis on mathematics must equal the attention to input (measurements).
Related products
Whole body room calorimeters
The Room Calorimeter offers the highest validated accuracy and reproducibility in the market. Designed on a system level out of the highest quality components, this is the gold standard for energy expenditure studies of any kind; 24-hr energy expenditure, high intensity exercise testing and many more. Validated and applied in 100’s of research studies.
How can we help you with your research?
Maastricht Instruments creates equipment in the field for indirect calorimetry measurements. We provide support for studies, research and measurements alongside our indirect calorimetry products.
Consult us about our indirect calorimetry metabolic cart, whole room calorimeter systems or accelerometry add-ons. Please contact us or find more information on our information pages.
Reference
Schoffelen, P.F.M., Plasqui, G. Classical experiments in whole-body metabolism: open-circuit respirometry—diluted flow chamber, hood, or facemask systems. Eur J Appl Physiol 118, 33–49 (2018). https://doi.org/10.1007/s00421-017-3735-5
